Background:
Development of ABL1 kinase domain (KD) mutation is one of resistance mechanism to ATP-binding pocket inhibitor (ABPI) therapy. T315I mutation in ABL KD confers broad-spectrum resistance to all 1st- and 2nd-generation ABPIs including Imatinib (IMA), Dasatinib (DAS), Nilotinib (NIL) and Bosutinib (BOS). Ponatinib (PON), a 3 rd generation inhibitor, can overcome T315I-mediated resistance in chronic myeloid leukemia (CML) treatment. However, its increased risk of cardiovascular toxicity limits the broader use of PON in the clinic. Asciminib (ASC) is a Specifically Targeting ABL1 Myristoyl Pocket (STAMP) inhibitor and has been approved for CML treatment in the patients who failed at least two previous lines of TKI therapy. ASC, which blocks the myristoylate pocket in ABL1 KD protein, can potentially overcome ABL1 KD mutation (KDM)-mediated tyrosine kinase inhibitor (TKI) resistance by combining with ABPIs which blocks ATP binding pockets in ABL1 KD protein. However, in vitro evidence to support double blockade approach is still to be further investigated including combination with ABPIs as well with other drug compounds.
Axitinib (AXI), which is approved for the treatment of advanced renal cell carcinoma (RCC), is an ABPI that can specifically bind to the ATP-binding pocket in ABL1 KD protein structurally harboring the T315I mutation. In the Cancer Therapeutics Response Portal (CTRP) database, which integrates drug compound and cell line experiment databases, AXI showed very low IC 50 in CML cell lines compared to other cancer cell lines, including RCC. The present study attempted to provide in vitro evidence of the synergistic enhancement of therapeutic efficacy of AXI combined with ASC to overcome T315I mutated CML.
Methods and Materials:
We analyzed IC 50 values for 649 cell lines tested with AXI using the CTRPv2 database. The drug response of AXI in K562/Wild-Type (WT), K562/T315I mut, BaF3/WT, and BaF3/T315I mut cell lines was evaluated using the WST-8 assay. From the measured IC 50 value in those cell lines as the baseline concentration (conc), AXI and ASC conc were serially diluted. The dose-response matrix and ZIP synergy score were analyzed using SynergyFinder.
Results
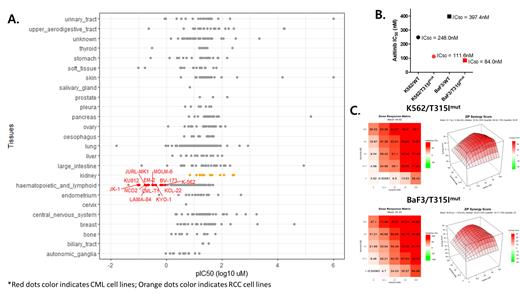
We compared the sensitivity of 649 different cell lines to AXI in the CTRPv2 database and confirmed that the IC 50 of AXI in CML (median IC 50 = 0.31 uM) is 96 times lower than that of various cell lines derived from RCC (median IC 50 = 29.85 uM) (Fig. 1A). This result implies that AXI is more effectively inhibit CML cells at a lower conc than RCC cell lines or other cell lines.
When measuring the inhibitory activityof AXI (Fig. 1B), AXI treatment showed 2.22 times lower IC 50 in the K562/T315I mut (IC 50 = 111.6nM) than in the K562/WT (IC 50 = 248nM). Also AXI treatment showed 4.73 times lower IC 50 in the BaF3/T315I mut (IC 50 = 84nM) than in the BaF3/WT (IC 50 = 397.4nM). Overall, the T315I carrying CML cell lines were much more sensitive to lower dose AXI than wild type CML cell lines.
In the dose-response matrix analysis (Fig. 1C), AXI treatment alone at 100 nM inhibited the growth of the K562/T315 mut cell line by 38.42%, and ASC treatment alone at 100 nM, by 36.52%. Of note, the combination of AXI at 25nM and ASC at 12.5nM inhibited cell growth by 38.1%, and the combination of AXI at 12.5nM and ASC at 25nM showed 38.42% inhibition, which is similar to the inhibition achieved by 100nM of each drug alone. The result suggests that the combination of reduced dose of each drug, by 25% or 12.5% from the baseline IC 50 dose, showed equivalent inhibitor activity to the baseline IC 50 concentration of each drug monotherapy in the K562/T315 mut cell line. Similarly, when AXI and ASC were combined at a reduced concentration by a half or a quarter and tested in the BaF3/T315I mut cell line, a higher inhibitory activity was observed compared to the baseline IC 50 concentration of each drug monotherapy. The ZIP score was calculated as 31.7 and 20.64 in K562/T315 mut and BaF3/T315I mut cell line. Given that ZIP score was above 10 each, synergistic enhancement of inhibitory activity was demonstrated against T315I mutant CML cells between AXI and ASC.
Conclusion:
This result suggests synergistically enhanced inhibitory activity of dual blockade using AXI combined with ASC specifically for T315I mutant CML. This approach will be promising against CML cells carrying compound mutation with T315I mutation, which is highly resistant even to Ponatinib or Asciminib.
Disclosures
Kimura:OHARA Pharmaceutical Co., Ltd.: Honoraria, Patents & Royalties, Research Funding. Kim:Sanofi: Consultancy, Honoraria; Merk: Consultancy; Paladin: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Novartis: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal